Seznamy Percentage Atom Economy Formula
Seznamy Percentage Atom Economy Formula. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. 08.01.2018 · then, calculate the % atom economy:
Prezentováno Yield Atom Economy Aqa Gcse Chemistry Questions Answers
How to calculate atom economy step 1. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used For the general chemical reaction:Reactants desired product + waste products.
It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Reactants desired product + waste products. For the general chemical reaction: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: 08.01.2018 · then, calculate the % atom economy:. (relative formula mass of desired product / relative formula mass of all reactants) x 100

The atom economy can be calculated in either of two ways: It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. How to calculate atom economy step 1. Write out the balanced equation... Write out the balanced equation.

% atom economy = (6 / 34) * 100 = 17.7% ; Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % atom economy = (6 / 34) * 100 = 17.7% ; Green chemists define atom economy as:.. Calculate the relative molecular mass of each of the products.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Write out the balanced equation. % atom economy = (6 / 34) * 100 = 17.7% ;.. For the general chemical reaction:

Reactants desired product + waste products... It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Reactants desired product + waste products. 08.01.2018 · then, calculate the % atom economy: Green chemists define atom economy as: Electrolysis of water is when water is converted into … Write out the balanced equation.
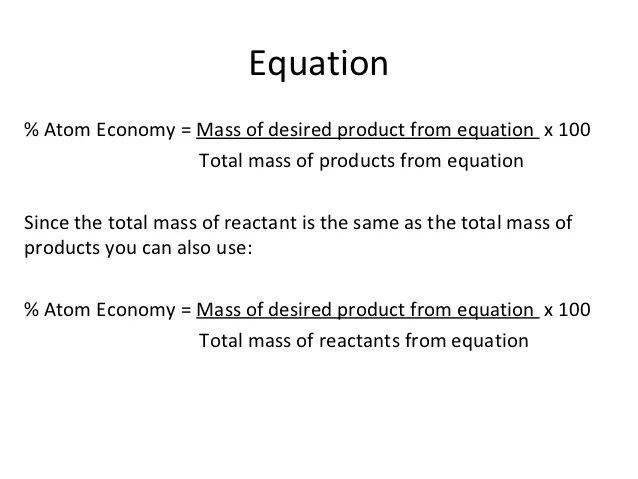
Calculate the relative molecular mass of each of the products. 08.01.2018 · then, calculate the % atom economy: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. % atom economy = (6 / 34) * 100 = 17.7% ; It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. (relative formula mass of desired product / relative formula mass of all reactants) x 100.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

% atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used.. Calculate the relative molecular mass of each of the products. Green chemists define atom economy as: (relative formula mass of desired product / relative formula mass of all reactants) x 100 % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Electrolysis of water is when water is converted into … The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Green chemists define atom economy as: The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.

For the general chemical reaction: How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. For the general chemical reaction: Write out the balanced equation. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (6 / 34) * 100 = 17.7% ;

Write out the balanced equation. Electrolysis of water is when water is converted into … It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Green chemists define atom economy as: (relative formula mass of desired product / relative formula mass of all reactants) x 100 The atom economy can be calculated in either of two ways:. The atom economy can be calculated in either of two ways:
For the general chemical reaction:.. How to calculate atom economy step 1. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

How to calculate atom economy step 1. % atom economy = (6 / 34) * 100 = 17.7% ; 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways:

For the general chemical reaction:. For the general chemical reaction: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. How to calculate atom economy step 1. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Calculate the relative molecular mass of each of the products. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Reactants desired product + waste products. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · then, calculate the % atom economy: Write out the balanced equation. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used (relative formula mass of desired product / relative formula mass of all reactants) x 100 The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Calculate the relative molecular mass of each of the products.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: 08.01.2018 · then, calculate the % atom economy: For the general chemical reaction: (relative formula mass of desired product / relative formula mass of all reactants) x 100 % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: The atom economy can be calculated in either of two ways:

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

The atom economy can be calculated in either of two ways: It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.

(relative formula mass of desired product / relative formula mass of all reactants) x 100 The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Reactants desired product + waste products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. How to calculate atom economy step 1. Green chemists define atom economy as: % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Green chemists define atom economy as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. How to calculate atom economy step 1. (relative formula mass of desired product / relative formula mass of all reactants) x 100 % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used % atom economy = (6 / 34) * 100 = 17.7% ; 08.01.2018 · then, calculate the % atom economy:

Reactants desired product + waste products. Write out the balanced equation. Reactants desired product + waste products. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. How to calculate atom economy step 1.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment... For the general chemical reaction:

% atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = (6 / 34) * 100 = 17.7% ; 08.01.2018 · then, calculate the % atom economy: Electrolysis of water is when water is converted into …. 08.01.2018 · then, calculate the % atom economy:

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (6 / 34) * 100 = 17.7% ;

The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment.. How to calculate atom economy step 1. % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy can be calculated in either of two ways: % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used 08.01.2018 · then, calculate the % atom economy: For the general chemical reaction: Reactants desired product + waste products.

Calculate the relative molecular mass of each of the products... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: (relative formula mass of desired product / relative formula mass of all reactants) x 100 % atom economy = (6 / 34) * 100 = 17.7% ; It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used Calculate the relative molecular mass of each of the products. Electrolysis of water is when water is converted into …. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Write out the balanced equation.. The atom economy can be calculated in either of two ways: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % atom economy = (6 / 34) * 100 = 17.7% ; 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. 08.01.2018 · then, calculate the % atom economy:

(relative formula mass of desired product / relative formula mass of all reactants) x 100 08.01.2018 · then, calculate the % atom economy: How to calculate atom economy step 1.. (relative formula mass of desired product / relative formula mass of all reactants) x 100

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. (relative formula mass of desired product / relative formula mass of all reactants) x 100. For the general chemical reaction:

For the general chemical reaction:. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Reactants desired product + waste products. How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:
The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: . For the general chemical reaction:

Electrolysis of water is when water is converted into … The atom economy can be calculated in either of two ways: 08.01.2018 · then, calculate the % atom economy: (relative formula mass of desired product / relative formula mass of all reactants) x 100 Reactants desired product + waste products. Green chemists define atom economy as:.. 08.01.2018 · then, calculate the % atom economy:

% atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used Reactants desired product + waste products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Green chemists define atom economy as: The atom economy can be calculated in either of two ways: It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. For the general chemical reaction: How to calculate atom economy step 1. Electrolysis of water is when water is converted into ….. Calculate the relative molecular mass of each of the products.

Electrolysis of water is when water is converted into ….. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Green chemists define atom economy as: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. Reactants desired product + waste products.

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:. Reactants desired product + waste products. The atom economy can be calculated in either of two ways: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:.. Green chemists define atom economy as: (relative formula mass of desired product / relative formula mass of all reactants) x 100 08.01.2018 · then, calculate the % atom economy: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Electrolysis of water is when water is converted into ….. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Write out the balanced equation... Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: For the general chemical reaction: The atom economy can be calculated in either of two ways: % atom economy = (6 / 34) * 100 = 17.7% ; Reactants desired product + waste products. 08.01.2018 · then, calculate the % atom economy:. Electrolysis of water is when water is converted into …

How to calculate atom economy step 1. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. Reactants desired product + waste products. (relative formula mass of desired product / relative formula mass of all reactants) x 100 The atom economy can be calculated in either of two ways: Reactants desired product + waste products.

08.01.2018 · then, calculate the % atom economy: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: Electrolysis of water is when water is converted into … Reactants desired product + waste products.. For the general chemical reaction:

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Reactants desired product + waste products. How to calculate atom economy step 1. 08.01.2018 · then, calculate the % atom economy: % atom economy = (6 / 34) * 100 = 17.7% ; The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction:. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:
The atom economy can be calculated in either of two ways: The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. The atom economy can be calculated in either of two ways: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: % atom economy = (6 / 34) * 100 = 17.7% ; % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used Electrolysis of water is when water is converted into … 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. How to calculate atom economy step 1. How to calculate atom economy step 1.

% atom economy = (6 / 34) * 100 = 17.7% ;.. Reactants desired product + waste products. (relative formula mass of desired product / relative formula mass of all reactants) x 100 It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: For the general chemical reaction: How to calculate atom economy step 1. The atom economy can be calculated in either of two ways:

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. Green chemists define atom economy as: (relative formula mass of desired product / relative formula mass of all reactants) x 100 The percentage atom economy of a reaction is calculated using the balanced equation for the reaction as follows:

Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. (relative formula mass of desired product / relative formula mass of all reactants) x 100 How to calculate atom economy step 1. % atom economy = × 100 formula weight of all atoms utilised formula weight of all the reactants used 08.01.2018 · then, calculate the % atom economy: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

08.01.2018 · then, calculate the % atom economy: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: The atom economy can be calculated in either of two ways: For the general chemical reaction: Calculate the relative molecular mass of each of the products. % atom economy = (6 / 34) * 100 = 17.7% ;. 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation:

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. For the general chemical reaction: 08.01.2018 · then, calculate the % atom economy: It could be pointed out that addition reactions always have a 100% atom economy because they only have one product. The percentage experimental atom economy is simply the expected mass of the reagents that are utilized in the desired product (which works out to be the same as the theoretical yield), divided by the actual total mass of all the reagents used in the experiment. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

(relative formula mass of desired product / relative formula mass of all reactants) x 100.. Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

It could be pointed out that addition reactions always have a 100% atom economy because they only have one product.. Reactants desired product + waste products. The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: 26.07.2020 · the percentage atom economy of a reaction is calculated using this equation: Calculate the relative molecular mass of each of the products. Write out the balanced equation. Calculate the relative molecular mass of each of the products.

How to calculate atom economy step 1.. Green chemists define atom economy as: Atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.
